Selected Publications
Detection and validation of novel mutations in MERTK in a simplex case of retinal degeneration using WGS and hiPSC–RPEs model
https://doi.org/10.1002/humu.24146
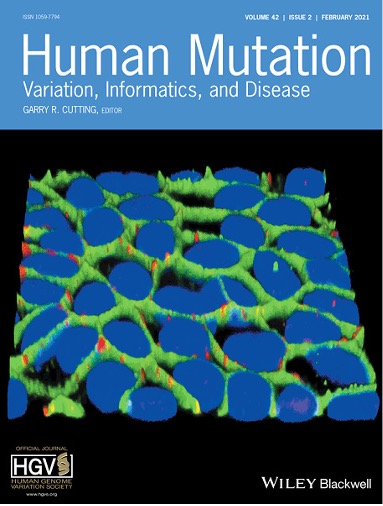
HiPSC-RPEs were generated from human peripheral blood cells. In the image the three-dimensional immunocytochemistry of hiPSC-RPE shows the localization of tight junction marker ZO-1 (green) and MERTK (red) proteins in polarized hiPSC-RPE. The nuclei are stained with DAPI in blue.
Pooja Biswas, Shyamanga Borooah, Hiroko Matsui, Marina Voronchikhina, Jason Zhou, Qais Zawaydeh, Pongali B. Raghavendra, Henry Ferreyra, S. Amer Riazuddin, Karl Wahlin, Kelly A. Frazer, Radha Ayyagari. Detection and validation of novel mutations in MERTK in a simplex case of retinal degeneration using WGS and hiPSC–RPEs model. Human Mutation. 2021; 42: 189– 199.
https://doi.org/10.1093/hmg/ddw113
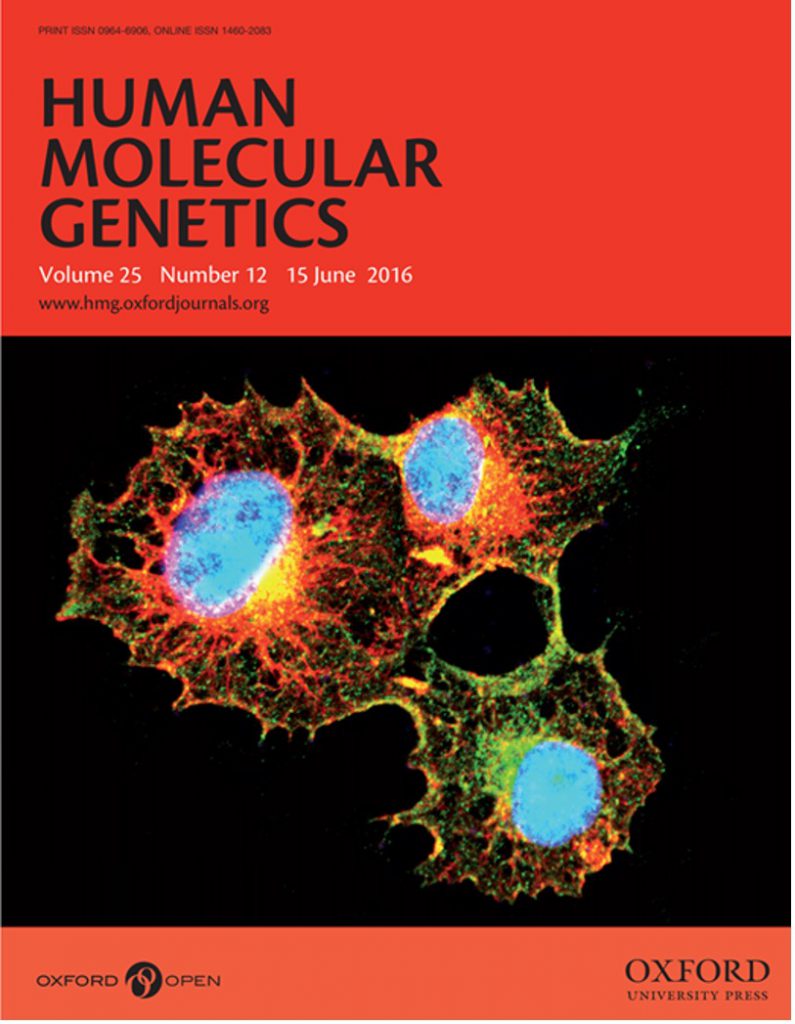
ASRGL1 is a novel gene associated with retinal degeneration. The image shows Cos-7 cells transfected with wild-type ASRGL1 tagged with cMyc (Alexa Fluor 488), which showed normal distribution of wild-type ASRGL1-cMyc fusion protein throughout the cytoplasm. The intracellular localization of wild-type ASRGL1 protein was compared relative to the cytoskeletal marker vimentin (Alexa Fluor 555).
Pooja Biswas, Venkata Ramana Murthy Chavali, Giulia Agnello, Everett Stone, Christina Chakarova, Jacque L. Duncan, Chitra Kannabiran, Melissa Homsher, Shomi S. Bhattacharya, Muhammad Asif Naeem, Adva Kimchi, Dror Sharon, Takeshi Iwata, Shaikh Riazuddin, G. Bhanuprakash Reddy, J. Fielding Hejtmancik, George Georgiou, S. Amer Riazuddin, Radha Ayyagari, A missense mutation in ASRGL1 is involved in causing autosomal recessive retinal degeneration, Human Molecular Genetics, Volume 25, Issue 12, 15 June 2016, Pages 2483–2497,
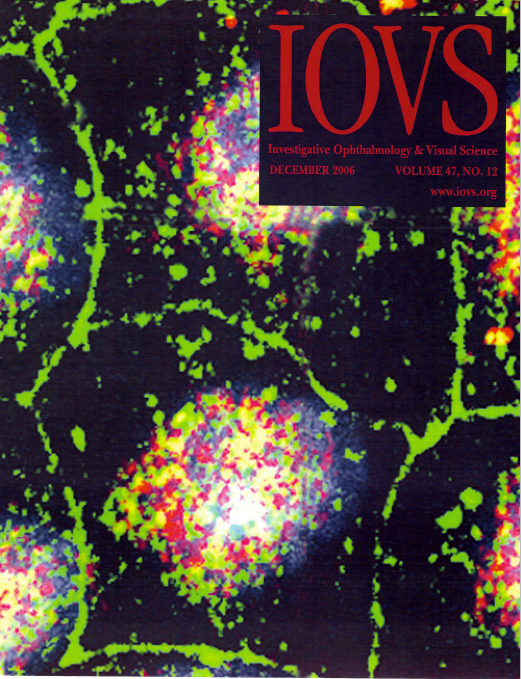
CTRP5 Is a Membrane-Associated and Secretory Protein in the RPE and Ciliary Body and the S163R Mutation of CTRP5 Impairs Its Secretion
https://doi.org/10.1167/iovs.06-0312.
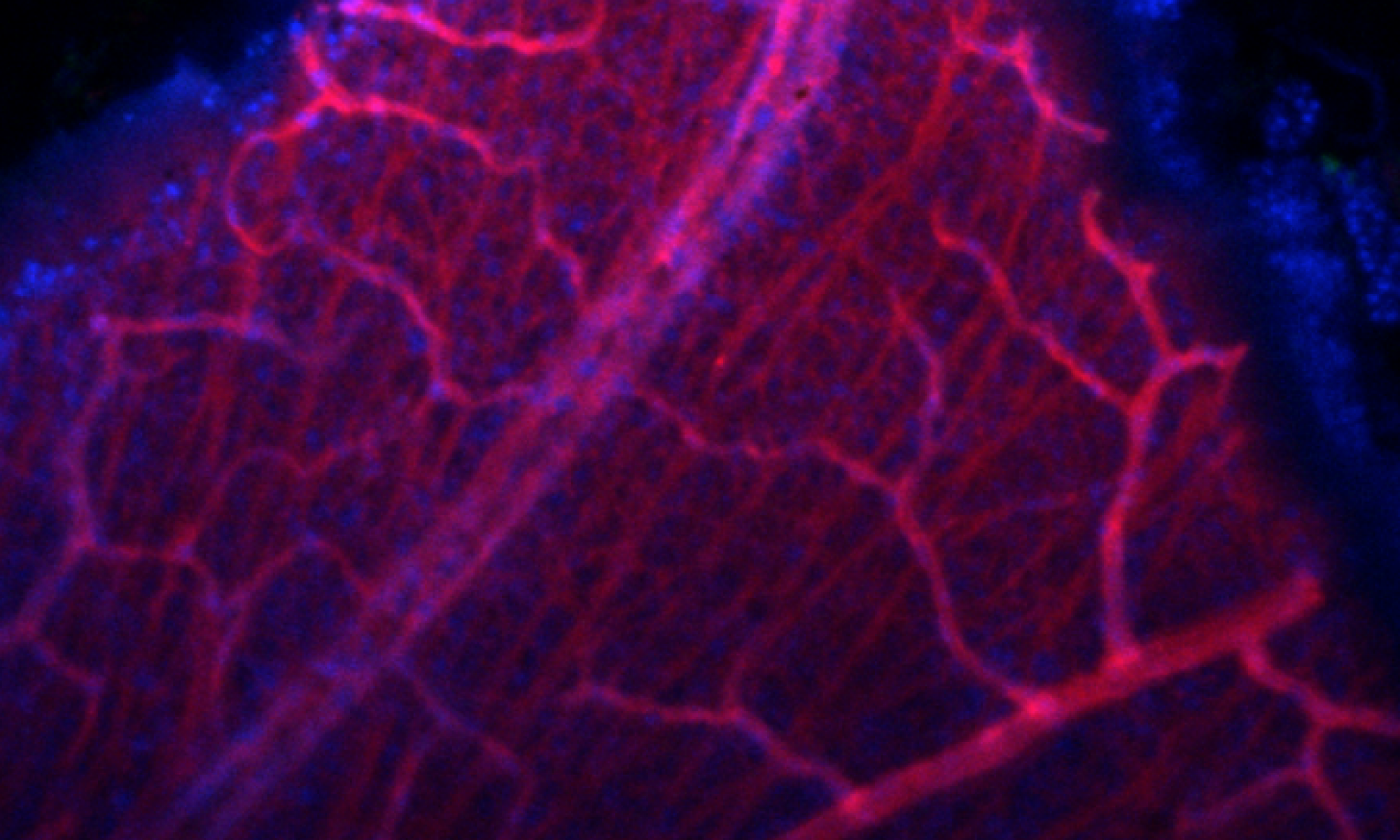
Distribution of MFRP and CTRP5 proteins in MDCK cells. Localization of MFRP protein is detected on the apical and lateral membrane locations (green). Significant co-localization of MFRP and CTRP5 proteins is observed on apical membranes (yellow).
Md Nawajes A. Mandal, Vidyullatha Vasireddy, G. Bhanuprakash Reddy, XiaoFei Wang, Sayoko E. Moroi, Bikash R. Pattnaik, Bret A. Hughes, John R. Heckenlively, Peter F. Hitchcock, Monica M. Jablonski, Radha Ayyagari; CTRP5 Is a Membrane-Associated and Secretory Protein in the RPE and Ciliary Body and the S163R Mutation of CTRP5 Impairs Its Secretion. Invest. Ophthalmol. Vis. Sci. 2006; 47(12):5505-5513.

Atrophic macular degeneration mutations in ELOVL4 result in the intracellular misrouting of the protein
https://doi.org/10.1016/j.ygeno.2003.10.004.
Localization of ELOVL4 to the inner segments of photoreceptors in the human retina. This protein is predicted to be localized to the endoplasmic reticulum. The image shows co-localization of ELOVL4 with the endoplasmic reticulum resident protein, disulphide isomerase. Mutations in the ELOVL4 gene cause dominant atrophic macular degeneration.
Rajesh Ambasudhan, XiaoFei Wang, Monica M Jablonski, Debra A Thompson, Pamela S Lagali, Paul W Wong, Paul A Sieving, Radha Ayyagari; Atrophic macular degeneration mutations in ELOVL4 result in the intracellular misrouting of the protein; Genomics, Volume 83, Issue 4, 2004, Pages 615-625; ISSN 0888-7543,
https://doi.org/10.1006/geno.2002.6815.
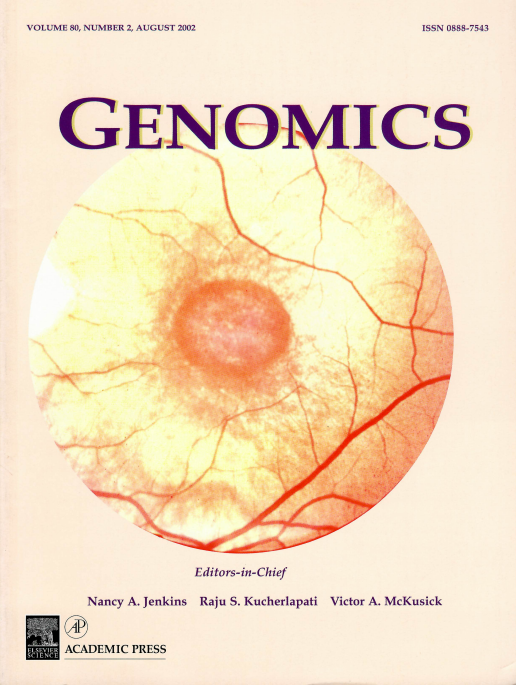
Retinal fungus photograph showing circumscribed macular degeneration in a 49-year-old affected male from a family in which X-linked recessive atrophic macular degeneration segregates with a RPGR gene mutation in ORF15.
Radha Ayyagari, F.Yesim Demirci, Jiafan Liu, Eve L. Bingham, Heather Stringham, Laura E. Kakuk, Michael Boehnke, Michael B. Gorin, Julia E. Richards, Paul A. Sieving; X-Linked Recessive Atrophic Macular Degeneration from RPGR Mutation; Genomics, Volume 80, Issue 2, 2002, Pages 166-171; ISSN 0888-7543,